The family of metal organic compounds called Grignard reagents (GR) are widely used in the chemical and pharmaceutical industry as a convenient way of creating C-C bonds.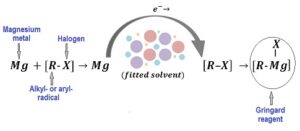
The solvent choice is really crucial – it coordinates and stabilizes the GR molecule.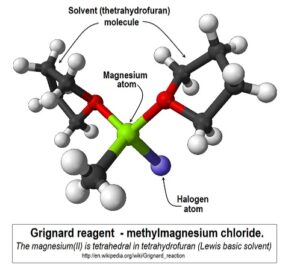
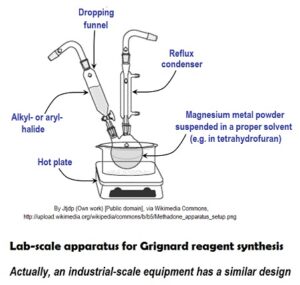
Commonly, Grignard reagents are synthesized by adding an organo-halide to magnesium metal powder suspended in a suitable dry, oxygen-free and un-reactive solvent.
Limitations and problems in established GR-synthesis process:
Magnesium powder is covered with passive oxide layer; this layer inhibits the Gringard reaction. The layer is to be removed (magnesium activation) for starting GR. Magnesium surface may activate and deactivate intermittently in course of Grignard reaction, though. This cause a serious runaway reaction risk because he Grignard reaction is strongly exothermic.
The most usable solvents are various ethers; these compounds are volatile, flammable and form explosive peroxides.
As a result, in spite of many years of industrial use, the commercial-scale production of GR has not been well established.
The new way of conducting of GR solves the above problems:
GR is performing in an ionic liquid.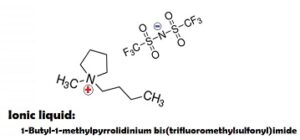
Ionic liquids comprised of 100% ions at room temperature; they are generally non-flammable, chemically stable, non-volatile, a great many are hydrophobic, and also have wide electrochemical windows.
Daniel Luder, Alexander Kraytsberg and Yair Ein-Eli, Catalyst-Free Electrochemical Grignard Reagent Synthesis with Room-Temperature Ionic Liquids, ChemElectroChem 2 (2014) 362 – 365
The process is patented.