Aluminum–air battery based on an ionic liquid electrolyte
Our society is in transition from a fossil fuel based economy to a clean energy economy for the past few years. This gradual but inevitable process is being accelerated by recent active research worldwide on sustainable energy harvesting, conversion and storage. Batteries have long been recognized for their capacity to efficiently store and convert chemical to electrical energy. They now find use in a myriad of applications extending from portable electronic devices, grid-scale energy storage to electric vehicles. Of the many different types of batteries marketed so far, Lithium-ion technology has dominated the consumer market since its advent by virtue of its high specific energy and power density. However, as needs progress and expand, new generations of devices requiring much more energy to support the ever increasing demands. These devices need smaller and lighter batteries containing much more energy than currently available technologies have to offer. Thus, a new chemistry, to support new battery technologies beyond Lithium-based must be developed.
One of the more promising avenues for achieving these targets are battery systems based on Aluminum (Al) and its alloys as the anodic material. Al, as the anodic material for such systems has many merits ranging low cost, derived from its naturally high abundance in the earth’s crust, ease of processing, high energy content, low equivalent weight to being non-toxic and environmental friendly. Al contains ~1/2 the energy of Gasoline per weight unit (8,100 Wh/kg for Al-air systems vs. 13,000 Wh/kg for Gasoline) and 3X the energy per volume unit (21,870 Wh/l for Al-air vs. 9,700 Wh/l for Gasoline). Currently, best practical utilization of Gasoline for automotive applications can reach approximately 1,700 Wh/kg where Al-air batteries using aqueous based electrolyte can reach 300-500 Wh/kg. However, current utilization of Al-air batteries using aqueous electrolytes, as a sustainable energy storage is hampered by two major problems:
- High corrosion rates of the Al anode, greatly reducing battery utilization and safety.
- Difficulty in Al surface activation causing a substantial limitation on power output.
Our study presents a novel non-aqueous Al–air battery. This battery utilizes 1-ethyl-3-
methylimidazolium oligo-fluoro-hydrogenate (EMIm(HF)2.3F) room temperature ionic liquid (RTIL). Al–air batteries can sustain current densities up to 1.5 mA cm-2, producing capacities above 140 mA h cm-2, thus utilizing above 70% of the theoretical Al capacity. This is equivalent to an outstanding energy densities of 2300 W h kg-1 and 6200 W h L-1. We detected Al2O3 at the air electrode as the battery discharge product of the oxygen reduction coupled with Al ions migrated to the air electrode.
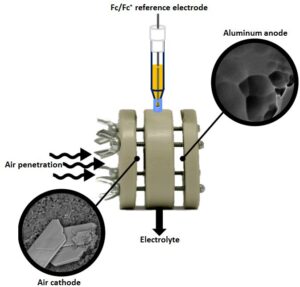
Benefits of this novel battery:
- More energy at less volume and less weight than state-of-the-art primary batteries
- Robust – no humidity issues, no CO2 consumption
- Extremely high utilization of the Aluminum anode
- Safe – Nonflammable, non-explosive
- Lower cost – Uses an inexpensive and abundant metal, longer shelf life
- Environment friendly: Easily recyclable, nontoxic, safe, clean energy
- Easily Processable – Printable, flexible


