The most important battery parameters are specific energy and energy density
The battery should also be safe and low cost; at the same time, the improvement of batteries with high energy performance is apparently more promising avenue of development than attempts to increase energy performance of cheap and safe batteries.
The clue to the cell energy performance is the cell chemistry, because
[Stored energy E] = [Stored charge Q] × [Cell voltage V]
From this standpoint, Li-based battery chemistry is the most promising since lithium metal is the most electropositive (E = -3.04 V vs. standard hydrogen electrode) and light (ρ = 0.53 gram per milliliter) among other materials.
However, employing lithium metal anode in a secondary cell is problematic because of the safety reasons (possible dendrite growth poses risks of anode-cathode shorting).
The introduction of the Li+ intercalation materials is a critical improvement of the Li-chemistry cells. Such materials have an ability to room Li-ions inside its crystal lattice.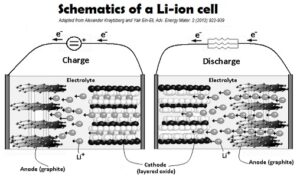
In case of the anode Li-ions are in “almost atomic” state, with nearly 100% charge transfer from the host lattice toward Li-ions inside the material. Commonly such intercalation anodes are made of carbonaceous materials. As the matter of fact, such anodes have an intercalation potential very close to the potential of Li/Li+ couple.
In the same way a cathode Li-ion intercalation material rooms lithium ions in an “almost Li+“, fully ionized state. Commonly such intercalation cathodes comprised of mixed transition metal oxides.
Carbon-based anodes have the intercalation potential very close to the potential of Li/Li+ couple and, as the matter of fact, Li-ion battery voltage depends on the cathode material.


