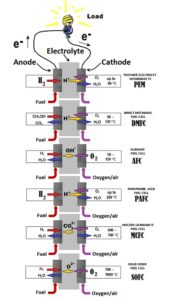
Fuel cells are an excellent energy conversion device for autonomous power supply – remote sites, transportation and backup power supply.
Different types of the fuel cells:
Fuel cells convert the chemical energy of fuel into electricity. As in batteries, fuel cells consist of an anode, where fuel oxidation occurs, and a cathode, where oxygen reduction occurs. An important part of the fuel cell is an electrolyte layer between cathode and anode. This material is conducting ions between the electrodes and is impermeable for electrons. Free electrons, which appear in course of fuel oxidation, are directed to the cathode through external circuit.
Fuel cells differ from batteries in the way that at the fuel and oxidant are not contained within the cell but are stored in the outside compartments. Fuel cells may be compared with internal combustion engines, as they operate as long as the fuel and oxygen are supplied. Fuel cells are usually not electrically rechargeable; instead, the fuel tank is to be refilled.