Phenomenological Passive-Active Transformation of Aluminum Surface in Ionic Liquid and its Beneficial Implementation in Batteries, B. Shvartsev, D. Gelman, D. Starosvetsky and Y. Ein-Eli, Langmuir, 31, 13860–13866 (2015).
Challenges and Prospect of Non-aqueous Non-alkali (NANA) Metal-Air Batteries, D. Gelman, B. Shvartsev, Y. Ein-Eli, Top. Curr. Chem., 374:82 (2016).
Li-air batteries
Li-air batteries are potentially viable ultrahigh energy density rechargeable chemical power sources, which could offer specific energies up to 3000Wh/kg being rechargeable.
Although Li/air cells hold the greatest promise in a number of applications ranging from portable electronics to electric vehicles, there are also impressive challenges in development of cathode materials and electrolyte systems of these
batteries.
The modern state of art and the challenges in the field of Li/air batteries are considered in our extensive review.
Non-aqueous Li-Air Battery: air cathode with a three-phase design
A new three phase design of the non-aqueous air cathode of Li-air battery
The cathode comprised of two interpenetrating and interconnected sub-systems of micro-channels
One system is filled with the electrolyte and another is filled with oxygen-transporting media, perfluorinated hydrocarbone liquid (PFC).
Oxygen freely diffuses through PFC-filled pore system while electrolyte-filled pore system transports metal ions to and from the cathode reaction sites enabling the oxygen reduction reaction.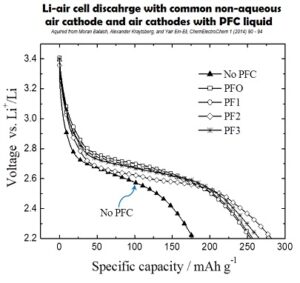
Li-air Battery – the electrolyte challenge
The decomposition of electrolyte and the considerably high rate of Li corrosion make it impossible to build Li-air cells with direct contact of lithium anode and aqueous electrolyte.
Several configurations are suggested for the Li-air system to avoid direct contact of Li-anode and aqueous electrolyte:
- Cells with fully non-aqueous liquid electrolytes, such as aprotic organic solvents and ionic liquids
All these designs are composed of a metallic lithium anode, an ionic conducting medium, and a porous air cathode commonly comprised of catalyst-coated carbon particles with large surface area.
Common non-aqueous organic electrolytes exhibit substantial capacity fading due to instability of the electrolyte caused by its volatility and parasitic reactions between the oxygen reduction species and organic electrolytes.
Some ionic liquids are stable against decomposition at an air cathode; however, currently employed ionic liquids have several shortcomings, i.e. low Li+ transference number, low Li-salt solubility and moisture susceptibility. These flaws impede practical use of ionic liquids for Li-air battery.
- Cells with solid state ion conducting medium
The solid state electrolytes made of Li-conducting glass ceramics or Li-conducting polymers are thought as a good alternative to the O2-attack- susceptible, volatile and flammable organic solvents. Such materials still suffer from low ionic conductivity.
- Mixed aqueous/non-aqueous electrolyte systems
In case of such design, a non-aqueous electrolyte is located at the anode side and aqueous electrolyte is located at the cathode side. In this configuration O2 diffuses into the porous carbon cathode where the oxygen reduction takes place, and lithium metal oxidizes into lithium ions which diffuse through the electrolyte interface into the cathode compartment.
The Li-metal anode protection from water penetration through the non-aqueous electrolyte layer is a critical issue for a long term stability of such Li-air cell.
A comprehensive review of the current state of art in the field of Li-air cell electrolyte systems is presented in our article.
SPE Li-Oxygen Battery Cell


