Today batteries based on LiCoO2 (LCO) and on LiNi0.8Co0.15Al0.05O2 (NCA) propel electric vehicles (EVs). One approach of improving the battery is replacing the cathode material, especially toward a material in the 5V range. Switching to a 5V cathode will enable to decrease the number of cells needed in today’s cars, thus reducing the weight, the volume and the cost of the battery and in addition increasing the battery’s energy. Those improvements will reduce the EVs cost and increase their driving range.
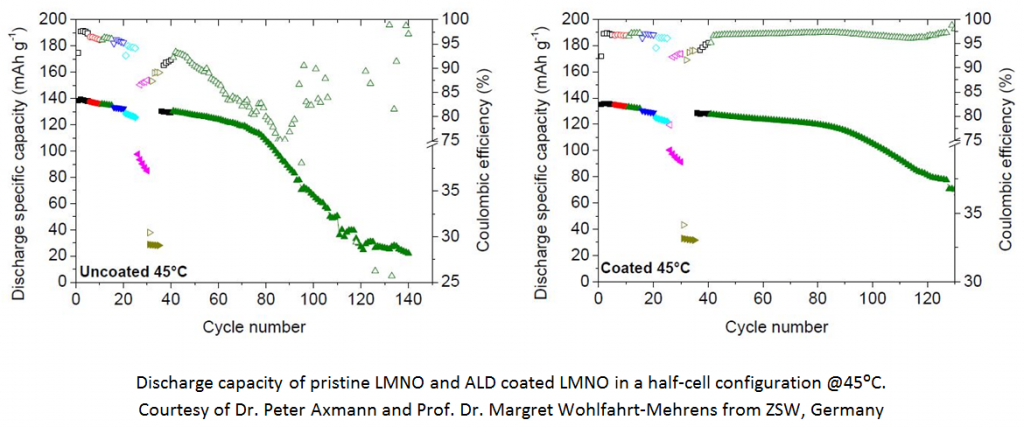
LiMn1.5Ni0.5O4 (LMNO) is an example of a cathode material with a voltage of 4.7V vs Li/Li+, which is well researched, unfortunately it is not yet commercial since it suffers from capacity fading which is very severe at elevated temperatures especially in the full cell configuration. The capacity fading is attributed to transition metal dissolution, mainly Mn2+ ions which dissolve into the electrolyte and precipitate on the anode.
Our approach to mitigate this challenge is to coat the cathode material in its powder form with metal fluorides using Atomic Layer Deposition (ALD), thus preventing or at least reducing substantially the amount of manganese ions dissolving into the electrolyte. Coating the powder with ALD enable us to control the coating layer thickness of each particle by changing the deposition temperature or by changing the number of deposition cycles.We have deposited MgF2 on LMNO powder and have shown improvement in the capacity retention in half-cell configuration.
Our recent study is focused on AlF3 deposition, which gives very promising results both in the half-cell configuration and more importantly in the full cell configuration.